Cell-Free Fetal DNA Testing Market - Forecast(2025 - 2031)
This report identifies the cell free fatal DNA testing market size in for the year 2014-2016, and forecast of the same for year 2021. It also highlights the potential growth opportunities in the coming years, while also reviewing the market drivers, restraints, growth indicators, challenges, market dynamics, competitive landscape, and other key aspects with respect to cell free fatal DNA testing market.
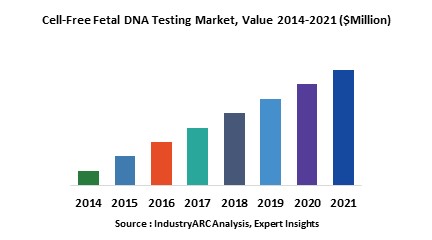
Globally North America dominated the market for cell free fatal DNA testing in 2015 due to developed medical infrastructure and high occurrence of several disease related to cell free DNA testing. North America is expected to continue its domination and was followed by Europe and Asia-Pacific as the second and third largest markets respectively in 2015. However Asia-Pacific is expected to be the fastest growing market due to technological advancement and changing medical infrastructure in the major economies in the region along with increasing awareness about necessity prenatal testing.
This report on global cell free fatal DNA testing also covers different type of cell free fatal DNA testing available, and market size in various geographical regions.
- On the basis of type of cell free fatal DNA testing this report classifies as follows covering all major types of cell free fatal DNA testing available in the market: Chromosome Number Abnormality Test, Paternal Inherited Disorder Test, Gender Test and Others
- This report has been further segmented into major regions, which includes detailed analysis of each region such as North America, Europe, Asia-Pacific (APAC) and Rest of the World (RoW) covering all the major country level markets for cell free fatal DNA testing in each of the region.
Sample Companies Profiled in this Report are:
- The Fetal Medicine Centre
- Apollo Path labs
- Ariosa Diagnostics
- Sequenom Laboratories
- Laboratory Corp. of America Holdings
- 10+
Key Market Players:
The Top 5 companies in Cell-Free Fetal DNA Testing Market are:
- Illumia Inc
- Thermo Fisher Scientific Inc.
- Natera
- Agilent Technologies Inc
- Yourgene Health
1. Cell-Free Fetal DNA Testing – Market Overview
2. Executive Summary
3. Cell-Free Fetal DNA Testing Market Landscape
3.1. Market Share Analysis
3.2. Comparative Analysis
3.2.1. Product Benchmarking
3.2.2. End User Profiling
3.2.3. Top 5 Financials Analysis
4. Cell-Free Fetal DNA Testing – Market Forces
4.1. Drivers
4.1.1. Increasing awareness about inherited diseases
4.1.2. Safe and low risk obstetric procedures for prenatal testing
4.2. Restraints
4.2.1. High Cost
4.3. Opportunities
4.4. Challenges
4.5. Porter’s Five Forces Analysis
4.5.1. Bargaining Power of Suppliers
4.5.2. Bargaining Power of Buyers
4.5.3. Threat of New Entrants
4.5.4. Threat of Substitutes
4.5.5. Degree of Competition
5. Cell-Free Fetal DNA Testing Market – Strategic Analysis
5.1. Value Chain Analysis
5.2. Pricing Analysis
5.3. Opportunities Analysis
5.4. Product/Market Life Cycle Analysis
5.5. Suppliers and Distributors
6. Global Cell-Free Fetal DNA Testing Market, By Test Type
6.1. Chromosome Number Abnormality Test
6.2. Paternal Inherited Disorder Test
6.3. Gender Test
6.4. Others
7. Global Cell-Free Fetal DNA Testing Market, By Geography
7.1. Europe
7.1.1. Germany
7.1.2. France
7.1.3. Italy
7.1.4. Spain
7.1.5. Russia
7.1.6. U.K.
7.1.7. Rest of Europe
7.2. Asia Pacific
7.2.1. China
7.2.2. India
7.2.3. Japan
7.2.4. South Korea
7.2.5. Rest of Asia-Pacific
7.3. North America
7.3.1. U.S.
7.3.2. Canada
7.3.3. Mexico
7.4. Rest of the World (RoW)
7.4.1. Brazil
7.4.2. Rest of RoW
8. Cell-Free Fetal DNA Testing – Market Entropy
8.1. Expansion
8.2. Technological Developments
8.3. Merger & Acquisitions, and Joint Ventures
8.4. Supply- Contract
9. Company Profiles
9.1. The Fetal Medicine Centre
9.1.1. Introduction
9.1.2. Financials
9.1.3. Key Insights
9.1.4. Key Strategy
9.1.5. Product Portfolio
9.1.6. SWOT Analysis
9.2. Apollo Path labs
9.2.1. Introduction
9.2.2. Financials
9.2.3. Key Insights
9.2.4. Key Strategy
9.2.5. Product Portfolio
9.2.6. SWOT Analysis
9.3. Ariosa Diagnostics
9.3.1. Introduction
9.3.2. Financials
9.3.3. Key Insights
9.3.4. Key Strategy
9.3.5. Product Portfolio
9.3.6. SWOT Analysis
9.4. Sequenom Laboratories
9.4.1. Introduction
9.4.2. Financials
9.4.3. Key Insights
9.4.4. Key Strategy
9.4.5. Product Portfolio
9.4.6. SWOT Analysis
9.5. Laboratory Corp. of America Holdings
9.5.1. Introduction
9.5.2. Financials
9.5.3. Key Insights
9.5.4. Key Strategy
9.5.5. Product Portfolio
9.5.6. SWOT Analysis
9.6. Personal Genome Diagnostics
9.6.1. Introduction
9.6.2. Financials
9.6.3. Key Insights
9.6.4. Key Strategy
9.6.5. Product Portfolio
9.6.6. SWOT Analysis
9.7. Premaitha Health
9.7.1. Introduction
9.7.2. Financials
9.7.3. Key Insights
9.7.4. Key Strategy
9.7.5. Product Portfolio
9.7.6. SWOT Analysis
9.8. Berry Genomics Co. Ltd
9.8.1. Introduction
9.8.2. Financials
9.8.3. Key Insights
9.8.4. Key Strategy
9.8.5. Product Portfolio
9.8.6. SWOT Analysis
9.9. Roche Holdings AG
9.9.1. Introduction
9.9.2. Financials
9.9.3. Key Insights
9.9.4. Key Strategy
9.9.5. Product Portfolio
9.9.6. SWOT Analysis
9.10. Trovagene, Inc
9.10.1. Introduction
9.10.2. Financials
9.10.3. Key Insights
9.10.4. Key Strategy
9.10.5. Product Portfolio
9.10.6. SWOT Analysis
9.11. Illumia Inc
9.12. Thermo Fisher Scientific Inc.
9.13. Natera
9.14. Agilent Technologies Inc
9.15. Yourgene Health
*More than 10 Companies are profiled in this Research Report, Complete List available on Request*
"*Financials would be provided on a best efforts basis for private companies"
10. Appendix
10.1. Abbreviations
10.2. Sources
10.3. Research Methodology
10.4. Expert Insights
List of Tables:
Table 1: Cell-Free Fetal DNA Testing Market Overview 2023-2030
Table 2: Cell-Free Fetal DNA Testing Market Leader Analysis 2023-2030 (US$)
Table 3: Cell-Free Fetal DNA Testing Market Product Analysis 2023-2030 (US$)
Table 4: Cell-Free Fetal DNA Testing Market End User Analysis 2023-2030 (US$)
Table 5: Cell-Free Fetal DNA Testing Market Patent Analysis 2013-2023* (US$)
Table 6: Cell-Free Fetal DNA Testing Market Financial Analysis 2023-2030 (US$)
Table 7: Cell-Free Fetal DNA Testing Market Driver Analysis 2023-2030 (US$)
Table 8: Cell-Free Fetal DNA Testing Market Challenges Analysis 2023-2030 (US$)
Table 9: Cell-Free Fetal DNA Testing Market Constraint Analysis 2023-2030 (US$)
Table 10: Cell-Free Fetal DNA Testing Market Supplier Bargaining Power Analysis 2023-2030 (US$)
Table 11: Cell-Free Fetal DNA Testing Market Buyer Bargaining Power Analysis 2023-2030 (US$)
Table 12: Cell-Free Fetal DNA Testing Market Threat of Substitutes Analysis 2023-2030 (US$)
Table 13: Cell-Free Fetal DNA Testing Market Threat of New Entrants Analysis 2023-2030 (US$)
Table 14: Cell-Free Fetal DNA Testing Market Degree of Competition Analysis 2023-2030 (US$)
Table 15: Cell-Free Fetal DNA Testing Market Value Chain Analysis 2023-2030 (US$)
Table 16: Cell-Free Fetal DNA Testing Market Pricing Analysis 2023-2030 (US$)
Table 17: Cell-Free Fetal DNA Testing Market Opportunities Analysis 2023-2030 (US$)
Table 18: Cell-Free Fetal DNA Testing Market Product Life Cycle Analysis 2023-2030 (US$)
Table 19: Cell-Free Fetal DNA Testing Market Supplier Analysis 2023-2030 (US$)
Table 20: Cell-Free Fetal DNA Testing Market Distributor Analysis 2023-2030 (US$)
Table 21: Cell-Free Fetal DNA Testing Market Trend Analysis 2023-2030 (US$)
Table 22: Cell-Free Fetal DNA Testing Market Size 2023 (US$)
Table 23: Cell-Free Fetal DNA Testing Market Forecast Analysis 2023-2030 (US$)
Table 24: Cell-Free Fetal DNA Testing Market Sales Forecast Analysis 2023-2030 (Units)
Table 25: Cell-Free Fetal DNA Testing Market, Revenue & Volume, By Test Type, 2023-2030 ($)
Table 26: Cell-Free Fetal DNA Testing Market By Test Type, Revenue & Volume, By Chromosome Number Abnormality Test, 2023-2030 ($)
Table 27: Cell-Free Fetal DNA Testing Market By Test Type, Revenue & Volume, By Paternal Inherited Disorder Test, 2023-2030 ($)
Table 28: Cell-Free Fetal DNA Testing Market By Test Type, Revenue & Volume, By Gender Test, 2023-2030 ($)
Table 29: North America Cell-Free Fetal DNA Testing Market, Revenue & Volume, By Test Type, 2023-2030 ($)
Table 30: South america Cell-Free Fetal DNA Testing Market, Revenue & Volume, By Test Type, 2023-2030 ($)
Table 31: Europe Cell-Free Fetal DNA Testing Market, Revenue & Volume, By Test Type, 2023-2030 ($)
Table 32: APAC Cell-Free Fetal DNA Testing Market, Revenue & Volume, By Test Type, 2023-2030 ($)
Table 33: Middle East & Africa Cell-Free Fetal DNA Testing Market, Revenue & Volume, By Test Type, 2023-2030 ($)
Table 34: Russia Cell-Free Fetal DNA Testing Market, Revenue & Volume, By Test Type, 2023-2030 ($)
Table 35: Israel Cell-Free Fetal DNA Testing Market, Revenue & Volume, By Test Type, 2023-2030 ($)
Table 36: Top Companies 2023 (US$) Cell-Free Fetal DNA Testing Market, Revenue & Volume
Table 37: Product Launch 2023-2030 Cell-Free Fetal DNA Testing Market, Revenue & Volume
Table 38: Mergers & Acquistions 2023-2030 Cell-Free Fetal DNA Testing Market, Revenue & Volume
List of Figures:
Figure 1: Overview of Cell-Free Fetal DNA Testing Market 2023-2030
Figure 2: Market Share Analysis for Cell-Free Fetal DNA Testing Market 2023 (US$)
Figure 3: Product Comparison in Cell-Free Fetal DNA Testing Market 2023-2030 (US$)
Figure 4: End User Profile for Cell-Free Fetal DNA Testing Market 2023-2030 (US$)
Figure 5: Patent Application and Grant in Cell-Free Fetal DNA Testing Market 2013-2023* (US$)
Figure 6: Top 5 Companies Financial Analysis in Cell-Free Fetal DNA Testing Market 2023-2030 (US$)
Figure 7: Market Entry Strategy in Cell-Free Fetal DNA Testing Market 2023-2030
Figure 8: Ecosystem Analysis in Cell-Free Fetal DNA Testing Market 2023
Figure 9: Average Selling Price in Cell-Free Fetal DNA Testing Market 2023-2030
Figure 10: Top Opportunites in Cell-Free Fetal DNA Testing Market 2023-2030
Figure 11: Market Life Cycle Analysis in Cell-Free Fetal DNA Testing Market
Figure 12: GlobalBy Test Type Cell-Free Fetal DNA Testing Market Revenue, 2023-2030 ($)
Figure 13: Global Cell-Free Fetal DNA Testing Market - By Geography
Figure 14: Global Cell-Free Fetal DNA Testing Market Value & Volume, By Geography, 2023-2030 ($)
Figure 15: Global Cell-Free Fetal DNA Testing Market CAGR, By Geography, 2023-2030 (%)
Figure 16: North America Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 17: US Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 18: US GDP and Population, 2023-2030 ($)
Figure 19: US GDP – Composition of 2023, By Sector of Origin
Figure 20: US Export and Import Value & Volume, 2023-2030 ($)
Figure 21: Canada Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 22: Canada GDP and Population, 2023-2030 ($)
Figure 23: Canada GDP – Composition of 2023, By Sector of Origin
Figure 24: Canada Export and Import Value & Volume, 2023-2030 ($)
Figure 25: Mexico Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 26: Mexico GDP and Population, 2023-2030 ($)
Figure 27: Mexico GDP – Composition of 2023, By Sector of Origin
Figure 28: Mexico Export and Import Value & Volume, 2023-2030 ($)
Figure 29: South America Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 30: Brazil Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 31: Brazil GDP and Population, 2023-2030 ($)
Figure 32: Brazil GDP – Composition of 2023, By Sector of Origin
Figure 33: Brazil Export and Import Value & Volume, 2023-2030 ($)
Figure 34: Venezuela Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 35: Venezuela GDP and Population, 2023-2030 ($)
Figure 36: Venezuela GDP – Composition of 2023, By Sector of Origin
Figure 37: Venezuela Export and Import Value & Volume, 2023-2030 ($)
Figure 38: Argentina Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 39: Argentina GDP and Population, 2023-2030 ($)
Figure 40: Argentina GDP – Composition of 2023, By Sector of Origin
Figure 41: Argentina Export and Import Value & Volume, 2023-2030 ($)
Figure 42: Ecuador Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 43: Ecuador GDP and Population, 2023-2030 ($)
Figure 44: Ecuador GDP – Composition of 2023, By Sector of Origin
Figure 45: Ecuador Export and Import Value & Volume, 2023-2030 ($)
Figure 46: Peru Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 47: Peru GDP and Population, 2023-2030 ($)
Figure 48: Peru GDP – Composition of 2023, By Sector of Origin
Figure 49: Peru Export and Import Value & Volume, 2023-2030 ($)
Figure 50: Colombia Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 51: Colombia GDP and Population, 2023-2030 ($)
Figure 52: Colombia GDP – Composition of 2023, By Sector of Origin
Figure 53: Colombia Export and Import Value & Volume, 2023-2030 ($)
Figure 54: Costa Rica Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 55: Costa Rica GDP and Population, 2023-2030 ($)
Figure 56: Costa Rica GDP – Composition of 2023, By Sector of Origin
Figure 57: Costa Rica Export and Import Value & Volume, 2023-2030 ($)
Figure 58: Europe Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 59: U.K Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 60: U.K GDP and Population, 2023-2030 ($)
Figure 61: U.K GDP – Composition of 2023, By Sector of Origin
Figure 62: U.K Export and Import Value & Volume, 2023-2030 ($)
Figure 63: Germany Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 64: Germany GDP and Population, 2023-2030 ($)
Figure 65: Germany GDP – Composition of 2023, By Sector of Origin
Figure 66: Germany Export and Import Value & Volume, 2023-2030 ($)
Figure 67: Italy Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 68: Italy GDP and Population, 2023-2030 ($)
Figure 69: Italy GDP – Composition of 2023, By Sector of Origin
Figure 70: Italy Export and Import Value & Volume, 2023-2030 ($)
Figure 71: France Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 72: France GDP and Population, 2023-2030 ($)
Figure 73: France GDP – Composition of 2023, By Sector of Origin
Figure 74: France Export and Import Value & Volume, 2023-2030 ($)
Figure 75: Netherlands Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 76: Netherlands GDP and Population, 2023-2030 ($)
Figure 77: Netherlands GDP – Composition of 2023, By Sector of Origin
Figure 78: Netherlands Export and Import Value & Volume, 2023-2030 ($)
Figure 79: Belgium Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 80: Belgium GDP and Population, 2023-2030 ($)
Figure 81: Belgium GDP – Composition of 2023, By Sector of Origin
Figure 82: Belgium Export and Import Value & Volume, 2023-2030 ($)
Figure 83: Spain Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 84: Spain GDP and Population, 2023-2030 ($)
Figure 85: Spain GDP – Composition of 2023, By Sector of Origin
Figure 86: Spain Export and Import Value & Volume, 2023-2030 ($)
Figure 87: Denmark Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 88: Denmark GDP and Population, 2023-2030 ($)
Figure 89: Denmark GDP – Composition of 2023, By Sector of Origin
Figure 90: Denmark Export and Import Value & Volume, 2023-2030 ($)
Figure 91: APAC Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 92: China Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030
Figure 93: China GDP and Population, 2023-2030 ($)
Figure 94: China GDP – Composition of 2023, By Sector of Origin
Figure 95: China Export and Import Value & Volume, 2023-2030 ($) Cell-Free Fetal DNA Testing Market China Export and Import Value & Volume, 2023-2030 ($)
Figure 96: Australia Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 97: Australia GDP and Population, 2023-2030 ($)
Figure 98: Australia GDP – Composition of 2023, By Sector of Origin
Figure 99: Australia Export and Import Value & Volume, 2023-2030 ($)
Figure 100: South Korea Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 101: South Korea GDP and Population, 2023-2030 ($)
Figure 102: South Korea GDP – Composition of 2023, By Sector of Origin
Figure 103: South Korea Export and Import Value & Volume, 2023-2030 ($)
Figure 104: India Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 105: India GDP and Population, 2023-2030 ($)
Figure 106: India GDP – Composition of 2023, By Sector of Origin
Figure 107: India Export and Import Value & Volume, 2023-2030 ($)
Figure 108: Taiwan Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 109: Taiwan GDP and Population, 2023-2030 ($)
Figure 110: Taiwan GDP – Composition of 2023, By Sector of Origin
Figure 111: Taiwan Export and Import Value & Volume, 2023-2030 ($)
Figure 112: Malaysia Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 113: Malaysia GDP and Population, 2023-2030 ($)
Figure 114: Malaysia GDP – Composition of 2023, By Sector of Origin
Figure 115: Malaysia Export and Import Value & Volume, 2023-2030 ($)
Figure 116: Hong Kong Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 117: Hong Kong GDP and Population, 2023-2030 ($)
Figure 118: Hong Kong GDP – Composition of 2023, By Sector of Origin
Figure 119: Hong Kong Export and Import Value & Volume, 2023-2030 ($)
Figure 120: Middle East & Africa Cell-Free Fetal DNA Testing Market Middle East & Africa 3D Printing Market Value & Volume, 2023-2030 ($)
Figure 121: Russia Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 122: Russia GDP and Population, 2023-2030 ($)
Figure 123: Russia GDP – Composition of 2023, By Sector of Origin
Figure 124: Russia Export and Import Value & Volume, 2023-2030 ($)
Figure 125: Israel Cell-Free Fetal DNA Testing Market Value & Volume, 2023-2030 ($)
Figure 126: Israel GDP and Population, 2023-2030 ($)
Figure 127: Israel GDP – Composition of 2023, By Sector of Origin
Figure 128: Israel Export and Import Value & Volume, 2023-2030 ($)
Figure 129: Entropy Share, By Strategies, 2023-2030* (%) Cell-Free Fetal DNA Testing Market
Figure 130: Developments, 2023-2030* Cell-Free Fetal DNA Testing Market
Figure 131: Company 1 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 132: Company 1 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 133: Company 1 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 134: Company 2 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 135: Company 2 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 136: Company 2 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 137: Company 3 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 138: Company 3 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 139: Company 3 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 140: Company 4 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 141: Company 4 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 142: Company 4 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 143: Company 5 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 144: Company 5 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 145: Company 5 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 146: Company 6 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 147: Company 6 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 148: Company 6 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 149: Company 7 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 150: Company 7 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 151: Company 7 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 152: Company 8 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 153: Company 8 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 154: Company 8 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 155: Company 9 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 156: Company 9 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 157: Company 9 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 158: Company 10 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 159: Company 10 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 160: Company 10 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 161: Company 11 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 162: Company 11 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 163: Company 11 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 164: Company 12 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 165: Company 12 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 166: Company 12 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 167: Company 13 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 168: Company 13 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 169: Company 13 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 170: Company 14 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 171: Company 14 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 172: Company 14 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
Figure 173: Company 15 Cell-Free Fetal DNA Testing Market Net Revenue, By Years, 2023-2030* ($)
Figure 174: Company 15 Cell-Free Fetal DNA Testing Market Net Revenue Share, By Business segments, 2023 (%)
Figure 175: Company 15 Cell-Free Fetal DNA Testing Market Net Sales Share, By Geography, 2023 (%)
 Email
Email Print
Print

