Vascular Access Devices Market - Forecast(2025 - 2031)
The Global Vascular Access Device market is valued of $8506.51 million at a CAGR of 6% during the forecast period 2024 -2030. Government initiatives along with the growing awareness about kidney failures, diabetes and cancer are expected to be the main drivers of this vascular access device market. North-America vascular access device market accounted for over 42% of the total industry share in 2017 and the demand for vascular access devices to treat the chronic kidney disease is likely to drive vascular access devices market in this region. Diagnostic Testing Application of vascular access device market accounted for 40.24% of the total market share in 2017.
What is Vascular Access Device?
Vascular Access Device are medical equipment’s used to get ingress the blood stream to supply medicines, administer fluids, nutritional components and collection of blood vessels and its products. These vascular access tubes are made from latex, silicon and other substances.
What are the major applications of Vascular Access Device?
A Venous Access Device basically allows admixture of solution which consists of nutritional or medication components without inducing problems that may occur in an Intravenous. Using VAD reduces the stress, pain and discomfort of repeated injection of needle stirenge. VAD usage is specifically for patients suffering with prolonged illness and requires continuous medication such as chemotherapy. Very specifically PICC is required when fluids or medicines are causing irritation in the wall of the veins. Catheters is an only solution to deeply access the circulatory system during the hemodialysis process. electric transformer.
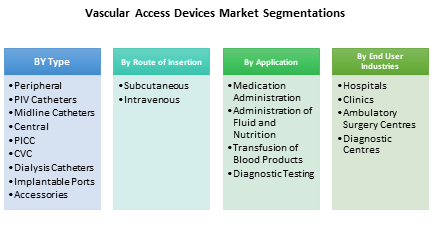
Market Research and Market Trends of Vascular Access Device
- Hospira Infusion System has been completely acquired by ICU Medical recently. This HIS consists of VAD, medical solutions and Intravenous pumps when joined with the company’s current business portfolio, will make ICU Medical as the world’s predominant player in the infusion therapy market. It is predicted that this acquisition gives ICU Medical a remarkable developed global impression and platform for long-term strength to compete and growth. With an Integrated product portfolio, the company become the leader in the market share and have in-depth access in the US Market.
- Needle is the key element in venous access device (VAD) for the infusion of medical fluids. But, recently needle-free VAD was introduced. Researchers stated that it would be the future generation of PIVO when it comes to enhance the experience of drawing blood from patient’s body, and lessen the uncertainty for both the practitioners and patients. This device will easy to use and will have high volume production. The FDA has been cleared for the usage of this device in US, which shows that there will be a huge impact in the PIVO market in US.
- Vascular Catheter devices is not only for the transfusion of blood and other medical fluids. Recently trended catheters such as Grid Mapping Catheters, which is sensor enabled device provides image capturing and data analysis in an impressive way to form a crystal clear maps of the heart valves to differentiate unhealthy and healthy tissues and other unwanted formation inside the heart valves. Since, it has unique features compared to other catheters, it is predicted that it would have huge demand in the medical applications.
Who are the Major Players in market?
The key players operating in the Vascular Access Device Market are C.R. Bard Inc., Navilyst Medical Inc., Terumo Corp., Becton., Smiths Medical Inc., Nipro Medical Corp., and other 10 more companies.
What is our report scope?
The report incorporates in-depth assessment of the competitive landscape, product market sizing, product benchmarking, market trends, product developments, financial analysis, strategic analysis and so on to gauge the impact forces and potential opportunities of the market. Apart from this the report also includes a study of major developments in the market such as product launches, agreements, acquisitions, collaborations, mergers and so on to comprehend the prevailing market dynamics at present and its impact during the forecast period 2014-2030.
All our reports are customizable to your company needs to a certain extent, we do provide 20 free consulting hours along with purchase of each report, and this will allow you to request any additional data to customize the report to your needs.
Key Takeaways from this Report
- Evaluate market potential through analyzing growth rates (CAGR %), Volume (Units) and Value ($M) data given at country level – for product types, end use applications and by different industry verticals.
- Understand the different dynamics influencing the market – key driving factors, challenges and hidden opportunities.
- Get in-depth insights on your competitor performance – market shares, strategies, financial benchmarking, product benchmarking, SWOT and more.
- Analyze the sales and distribution channels across key geographies to improve top-line revenues.
- Understand the industry supply chain with a deep-dive on the value augmentation at each step, in order to optimize value and bring efficiencies in your processes.
- Get a quick outlook on the market entropy – M&A’s, deals, partnerships, product launches of all key players for the past 4 years.
- Evaluate the supply-demand gaps, import-export statistics and regulatory landscape for more than top 20 countries globally for the market.
Key Market Players:
The Top 5 companies in Vascular Access Device Market are:
- Teleflex Inc.
- Medtronic PLC
- Angiodynamics Inc.
- Becton Dickinson & Co.
- B. Braun Melsungen AG
For more Lifesciences and Healthcare related reports, please click here
1. Vascular Access Devices Market - Overview
1.1. Definitions and Scope
2. Vascular Access Devices Market - Executive summary
2.1. Market Revenue, Market Size and Key Trends by Company
2.2. Key Trends by type of Application
2.3. Key Trends segmented by Geography
3. Vascular Access Devices Market
3.1. Comparative analysis
3.1.1. Product Benchmarking - Top 10 companies
3.1.2. Top 5 Financials Analysis
3.1.3. Market Value split by Top 10 companies
3.1.4. Patent Analysis - Top 10 companies
3.1.5. Pricing Analysis
4. Vascular Access Devices Market – Startup companies Scenario Premium
4.1. Top 10 startup company Analysis by
4.1.1. Investment
4.1.2. Revenue
4.1.3. Market Shares
4.1.4. Market Size and Application Analysis
4.1.5. Venture Capital and Funding Scenario
5. Vascular Access Devices Market – Industry Market Entry Scenario Premium
5.1. Regulatory Framework Overview
5.2. New Business and Ease of Doing business index
5.3. Case studies of successful ventures
5.4. Customer Analysis – Top 10 companies
6. Vascular Access Devices Market Forces
6.1. Drivers
6.2. Constraints
6.3. Challenges
6.4. Porters five force model
6.4.1. Bargaining power of suppliers
6.4.2. Bargaining powers of customers
6.4.3. Threat of new entrants
6.4.4. Rivalry among existing players
6.4.5. Threat of substitutes
7. Vascular Access Devices Market -Strategic analysis
7.1. Value chain analysis
7.2. Opportunities analysis
7.3. Product life cycle
7.4. Suppliers and distributors Market Share
8. Vascular Access Devices Market – By Route of Insertion (Market Size -$Million / $Billion)
8.1. Market Size and Market Share Analysis
8.2. Application Revenue and Trend Research
8.3. Product Segment Analysis
8.3.1. Subcutaneous
8.3.2. Intravenous
8.3.3. Intraosseous
9. Vascular Access Devices Market – By Type (Market Size -$Million / $Billion)
9.1. Introduction
9.2. Catheters
9.2.1. Arterial Access
9.2.2. Central Venous Access Device
9.2.2.1. Peripherally Inserted Central Catheters (PICC)
9.2.2.2. Tunneled Catheters
9.2.2.3. Non-Tunneled Catheters
9.2.2.4. Implanted Ports
9.2.3. Peripheral Venous Access Device
9.2.3.1. Peripheral Short Devices
9.2.3.2. Peripheral Midline Devices
9.2.3.3. Butterfly/Winged Steel Needles
9.2.4. Peripheral Catheters System
9.2.5. Midline Catheters
9.3. Vascular Access System
9.4. Sheath Introducers
9.5. Catheter Tip Positioning System.
9.9. Microcatheter
9.7. Balloon Occlusive Catheter
9.8. Guidewire
9.9. Guiding Sheath
9.10. Angiographic Catheter
9.11. Accessories
9.11.1. Safety Infusion Set
9.11.2. Transducer Cover Set
9.11.3. Coaxial Micro Introducer
9.11.4. Peelable Micro Introducer
9.12. Others
10. Vascular Access Devices Market – By Application (Market Size -$Million / $Billion)
10.1. Introduction
10.2. Medication Administration
10.3. Administration of Fluid and Nutrition
10.4. Transfusion of Blood Products
10.5. Diagnostic Testing
10.6. Drug Administration
11. Vascular Access Devices - By Geography (Market Size -$Million / $Billion)
11.1. Vascular Access Devices Market - North America Segment Research
11.2. North America Market Research (Million / $Billion)
11.2.1. Segment type Size and Market Size Analysis
11.2.2. Revenue and Trends
11.2.3. Application Revenue and Trends by type of Application
11.2.4. Company Revenue and Product Analysis
11.2.5. North America Product type and Application Market Size
11.2.5.1. U.S.
11.2.5.2. Canada
11.2.5.3. Mexico
11.2.5.4. Rest of North America
11.3. Vascular Access Devices - South America Segment Research
11.4. South America Market Research (Market Size -$Million / $Billion)
11.4.1. Segment type Size and Market Size Analysis
11.4.2. Revenue and Trends
11.4.3. Application Revenue and Trends by type of Application
11.4.4. Company Revenue and Product Analysis
11.4.5. South America Product type and Application Market Size
11.4.5.1. Brazil
11.4.5.2. Venezuela
11.4.5.3. Argentina
11.4.5.4. Ecuador
11.4.5.5. Peru
11.4.5.6. Colombia
11.4.5.7. Costa Rica
11.4.5.8. Rest of South America
11.5. Vascular Access Devices - Europe Segment Research
11.6. Europe Market Research (Market Size -$Million / $Billion)
11.6.1. Segment type Size and Market Size Analysis
11.6.2. Revenue and Trends
11.6.3. Application Revenue and Trends by type of Application
11.6.4. Company Revenue and Product Analysis
11.6.5. Europe Segment Product type and Application Market Size
11.6.5.1. U.K
11.6.5.2. Germany
11.6.5.3. Italy
11.6.5.4. France
11.6.5.5. Netherlands
11.6.5.6. Belgium
11.6.5.7. Spain
11.6.5.8. Denmark
11.6.5.9. Rest of Europe
11.7. Vascular Access Devices – APAC Segment Research
11.8. APAC Market Research (Market Size -$Million / $Billion)
11.8.1. Segment type Size and Market Size Analysis
11.8.2. Revenue and Trends
11.8.3. Application Revenue and Trends by type of Application
11.8.4. Company Revenue and Product Analysis
11.8.5. APAC Segment – Product type and Application Market Size
11.8.5.1. China
11.8.5.2. Australia
11.8.5.3. Japan
11.8.5.4. South Korea
11.8.5.5. India
11.8.5.6. Taiwan
11.8.5.7. Malaysia
12. Vascular Access Devices Market - Entropy
12.1. New product launches
12.2. M&A's, collaborations, JVs and partnerships
13. Vascular Access Devices Market – Industry / Segment Competition landscape Premium
13.1. Market Share Analysis
13.1.1. Market Share by Country- Top companies
13.1.2. Market Share by Region- Top 10 companies
13.1.3. Market Share by type of Application – Top 10 companies
13.1.4. Market Share by type of Product / Product category- Top 10 companies
13.1.5. Market Share at global level- Top 10 companies
13.1.6. Best Practices for companies
14. Vascular Access Devices Market – Key Company List by Country Premium
15. Vascular Access Devices Market Company Analysis
15.1. Market Share, Company Revenue, Products, M&A, Developments
15.2. C.R. Bard Inc
15.3. Navilyst Medical Inc
15.4. Terumo Corp
15.5. Becton
15.6. Smiths Medical Inc
15.7. Nipro Medical Corp
15.8. Teleflex Inc.
15.9. Medtronic PLC
15.10. Angiodynamics Inc.
15.11. B. Braun Melsungen AG
15.12. Becton Dickinson & Co.
"*Financials would be provided on a best efforts basis for private companies"
16. Vascular Access Devices Market - Appendix
16.1. Abbreviations
16.2. Sources
17. Vascular Access Devices Market - Methodology
17.1. Research Methodology
17.1.1. Company Expert Interviews
17.1.2. Industry Databases
17.1.3. Associations
17.1.4. Company News
17.1.5. Company Annual Reports
17.1.6. Application Trends
17.1.7. New Products and Product database
17.1.8. Company Transcripts
17.1.9. R&D Trends
17.1.10. Key Opinion Leaders Interviews
17.1.11. Supply and Demand Trends
List of Tables:
Table 1: Vascular Access Devices Market Overview 2023-2030
Table 2: Vascular Access Devices Market Leader Analysis 2023-2030 (US$)
Table 3: Vascular Access Devices Market Product Analysis 2023-2030 (US$)
Table 4: Vascular Access Devices Market End User Analysis 2023-2030 (US$)
Table 5: Vascular Access Devices Market Patent Analysis 2013-2023* (US$)
Table 6: Vascular Access Devices Market Financial Analysis 2023-2030 (US$)
Table 7: Vascular Access Devices Market Driver Analysis 2023-2030 (US$)
Table 8: Vascular Access Devices Market Challenges Analysis 2023-2030 (US$)
Table 9: Vascular Access Devices Market Constraint Analysis 2023-2030 (US$)
Table 10: Vascular Access Devices Market Supplier Bargaining Power Analysis 2023-2030 (US$)
Table 11: Vascular Access Devices Market Buyer Bargaining Power Analysis 2023-2030 (US$)
Table 12: Vascular Access Devices Market Threat of Substitutes Analysis 2023-2030 (US$)
Table 13: Vascular Access Devices Market Threat of New Entrants Analysis 2023-2030 (US$)
Table 14: Vascular Access Devices Market Degree of Competition Analysis 2023-2030 (US$)
Table 15: Vascular Access Devices Market Value Chain Analysis 2023-2030 (US$)
Table 16: Vascular Access Devices Market Pricing Analysis 2023-2030 (US$)
Table 17: Vascular Access Devices Market Opportunities Analysis 2023-2030 (US$)
Table 18: Vascular Access Devices Market Product Life Cycle Analysis 2023-2030 (US$)
Table 19: Vascular Access Devices Market Supplier Analysis 2023-2030 (US$)
Table 20: Vascular Access Devices Market Distributor Analysis 2023-2030 (US$)
Table 21: Vascular Access Devices Market Trend Analysis 2023-2030 (US$)
Table 22: Vascular Access Devices Market Size 2023 (US$)
Table 23: Vascular Access Devices Market Forecast Analysis 2023-2030 (US$)
Table 24: Vascular Access Devices Market Sales Forecast Analysis 2023-2030 (Units)
Table 25: Vascular Access Devices Market, Revenue & Volume, By Regulatory Process (Qualitative), 2023-2030 ($)
Table 26: Vascular Access Devices Market By Regulatory Process (Qualitative), Revenue & Volume, By Regulatory Issues/Recalls, 2023-2030 ($)
Table 27: Vascular Access Devices Market By Regulatory Process (Qualitative), Revenue & Volume, By Clinical Trail Analysis, 2023-2030 ($)
Table 28: Vascular Access Devices Market, Revenue & Volume, By Type, 2023-2030 ($)
Table 29: Vascular Access Devices Market By Type, Revenue & Volume, By Catheters, 2023-2030 ($)
Table 30: Vascular Access Devices Market By Type, Revenue & Volume, By Vascular Access System, 2023-2030 ($)
Table 31: Vascular Access Devices Market By Type, Revenue & Volume, By Sheath Introducers, 2023-2030 ($)
Table 32: Vascular Access Devices Market By Type, Revenue & Volume, By Catheter Tip Positioning Systems, 2023-2030 ($)
Table 33: Vascular Access Devices Market By Type, Revenue & Volume, By Accessories, 2023-2030 ($)
Table 34: Vascular Access Devices Market, Revenue & Volume, By Route of Insertion, 2023-2030 ($)
Table 35: Vascular Access Devices Market By Route of Insertion, Revenue & Volume, By Subcutaneous, 2023-2030 ($)
Table 36: Vascular Access Devices Market By Route of Insertion, Revenue & Volume, By Intravenous, 2023-2030 ($)
Table 37: Vascular Access Devices Market By Route of Insertion, Revenue & Volume, By Intraosseous, 2023-2030 ($)
Table 38: Vascular Access Devices Market, Revenue & Volume, By Application, 2023-2030 ($)
Table 39: Vascular Access Devices Market By Application, Revenue & Volume, By Medication Administration, 2023-2030 ($)
Table 40: Vascular Access Devices Market By Application, Revenue & Volume, By Administration of Fluid and Nutrition, 2023-2030 ($)
Table 41: Vascular Access Devices Market By Application, Revenue & Volume, By Transfusion of Blood Products, 2023-2030 ($)
Table 42: Vascular Access Devices Market By Application, Revenue & Volume, By Diagnostic Testing, 2023-2030 ($)
Table 43: Vascular Access Devices Market, Revenue & Volume, By End Users, 2023-2030 ($)
Table 44: Vascular Access Devices Market By End Users, Revenue & Volume, By Hospitals, 2023-2030 ($)
Table 45: Vascular Access Devices Market By End Users, Revenue & Volume, By Clinics, 2023-2030 ($)
Table 46: Vascular Access Devices Market By End Users, Revenue & Volume, By Ambulatory Surgery Centers, 2023-2030 ($)
Table 47: Vascular Access Devices Market By End Users, Revenue & Volume, By Diagnostic Centers, 2023-2030 ($)
Table 48: North America Vascular Access Devices Market, Revenue & Volume, By Regulatory Process (Qualitative), 2023-2030 ($)
Table 49: North America Vascular Access Devices Market, Revenue & Volume, By Type, 2023-2030 ($)
Table 50: North America Vascular Access Devices Market, Revenue & Volume, By Route of Insertion, 2023-2030 ($)
Table 51: North America Vascular Access Devices Market, Revenue & Volume, By Application, 2023-2030 ($)
Table 52: North America Vascular Access Devices Market, Revenue & Volume, By End Users, 2023-2030 ($)
Table 53: South america Vascular Access Devices Market, Revenue & Volume, By Regulatory Process (Qualitative), 2023-2030 ($)
Table 54: South america Vascular Access Devices Market, Revenue & Volume, By Type, 2023-2030 ($)
Table 55: South america Vascular Access Devices Market, Revenue & Volume, By Route of Insertion, 2023-2030 ($)
Table 56: South america Vascular Access Devices Market, Revenue & Volume, By Application, 2023-2030 ($)
Table 57: South america Vascular Access Devices Market, Revenue & Volume, By End Users, 2023-2030 ($)
Table 58: Europe Vascular Access Devices Market, Revenue & Volume, By Regulatory Process (Qualitative), 2023-2030 ($)
Table 59: Europe Vascular Access Devices Market, Revenue & Volume, By Type, 2023-2030 ($)
Table 60: Europe Vascular Access Devices Market, Revenue & Volume, By Route of Insertion, 2023-2030 ($)
Table 61: Europe Vascular Access Devices Market, Revenue & Volume, By Application, 2023-2030 ($)
Table 62: Europe Vascular Access Devices Market, Revenue & Volume, By End Users, 2023-2030 ($)
Table 63: APAC Vascular Access Devices Market, Revenue & Volume, By Regulatory Process (Qualitative), 2023-2030 ($)
Table 64: APAC Vascular Access Devices Market, Revenue & Volume, By Type, 2023-2030 ($)
Table 65: APAC Vascular Access Devices Market, Revenue & Volume, By Route of Insertion, 2023-2030 ($)
Table 66: APAC Vascular Access Devices Market, Revenue & Volume, By Application, 2023-2030 ($)
Table 67: APAC Vascular Access Devices Market, Revenue & Volume, By End Users, 2023-2030 ($)
Table 68: Middle East & Africa Vascular Access Devices Market, Revenue & Volume, By Regulatory Process (Qualitative), 2023-2030 ($)
Table 69: Middle East & Africa Vascular Access Devices Market, Revenue & Volume, By Type, 2023-2030 ($)
Table 70: Middle East & Africa Vascular Access Devices Market, Revenue & Volume, By Route of Insertion, 2023-2030 ($)
Table 71: Middle East & Africa Vascular Access Devices Market, Revenue & Volume, By Application, 2023-2030 ($)
Table 72: Middle East & Africa Vascular Access Devices Market, Revenue & Volume, By End Users, 2023-2030 ($)
Table 73: Russia Vascular Access Devices Market, Revenue & Volume, By Regulatory Process (Qualitative), 2023-2030 ($)
Table 74: Russia Vascular Access Devices Market, Revenue & Volume, By Type, 2023-2030 ($)
Table 75: Russia Vascular Access Devices Market, Revenue & Volume, By Route of Insertion, 2023-2030 ($)
Table 76: Russia Vascular Access Devices Market, Revenue & Volume, By Application, 2023-2030 ($)
Table 77: Russia Vascular Access Devices Market, Revenue & Volume, By End Users, 2023-2030 ($)
Table 78: Israel Vascular Access Devices Market, Revenue & Volume, By Regulatory Process (Qualitative), 2023-2030 ($)
Table 79: Israel Vascular Access Devices Market, Revenue & Volume, By Type, 2023-2030 ($)
Table 80: Israel Vascular Access Devices Market, Revenue & Volume, By Route of Insertion, 2023-2030 ($)
Table 81: Israel Vascular Access Devices Market, Revenue & Volume, By Application, 2023-2030 ($)
Table 82: Israel Vascular Access Devices Market, Revenue & Volume, By End Users, 2023-2030 ($)
Table 83: Top Companies 2023 (US$) Vascular Access Devices Market, Revenue & Volume
Table 84: Product Launch 2023-2030 Vascular Access Devices Market, Revenue & Volume
Table 85: Mergers & Acquistions 2023-2030 Vascular Access Devices Market, Revenue & Volume
List of Figures:
Figure 1: Overview of Vascular Access Devices Market 2023-2030
Figure 2: Market Share Analysis for Vascular Access Devices Market 2023 (US$)
Figure 3: Product Comparison in Vascular Access Devices Market 2023-2030 (US$)
Figure 4: End User Profile for Vascular Access Devices Market 2023-2030 (US$)
Figure 5: Patent Application and Grant in Vascular Access Devices Market 2013-2023* (US$)
Figure 6: Top 5 Companies Financial Analysis in Vascular Access Devices Market 2023-2030 (US$)
Figure 7: Market Entry Strategy in Vascular Access Devices Market 2023-2030
Figure 8: Ecosystem Analysis in Vascular Access Devices Market 2023
Figure 9: Average Selling Price in Vascular Access Devices Market 2023-2030
Figure 10: Top Opportunites in Vascular Access Devices Market 2023-2030
Figure 11: Market Life Cycle Analysis in Vascular Access Devices Market
Figure 12: GlobalBy Regulatory Process (Qualitative) Vascular Access Devices Market Revenue, 2023-2030 ($)
Figure 13: GlobalBy Type Vascular Access Devices Market Revenue, 2023-2030 ($)
Figure 14: GlobalBy Route of Insertion Vascular Access Devices Market Revenue, 2023-2030 ($)
Figure 15: GlobalBy Application Vascular Access Devices Market Revenue, 2023-2030 ($)
Figure 16: GlobalBy End Users Vascular Access Devices Market Revenue, 2023-2030 ($)
Figure 17: Global Vascular Access Devices Market - By Geography
Figure 18: Global Vascular Access Devices Market Value & Volume, By Geography, 2023-2030 ($)
Figure 19: Global Vascular Access Devices Market CAGR, By Geography, 2023-2030 (%)
Figure 20: North America Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 21: US Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 22: US GDP and Population, 2023-2030 ($)
Figure 23: US GDP – Composition of 2023, By Sector of Origin
Figure 24: US Export and Import Value & Volume, 2023-2030 ($)
Figure 25: Canada Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 26: Canada GDP and Population, 2023-2030 ($)
Figure 27: Canada GDP – Composition of 2023, By Sector of Origin
Figure 28: Canada Export and Import Value & Volume, 2023-2030 ($)
Figure 29: Mexico Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 30: Mexico GDP and Population, 2023-2030 ($)
Figure 31: Mexico GDP – Composition of 2023, By Sector of Origin
Figure 32: Mexico Export and Import Value & Volume, 2023-2030 ($)
Figure 33: South America Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 34: Brazil Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 35: Brazil GDP and Population, 2023-2030 ($)
Figure 36: Brazil GDP – Composition of 2023, By Sector of Origin
Figure 37: Brazil Export and Import Value & Volume, 2023-2030 ($)
Figure 38: Venezuela Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 39: Venezuela GDP and Population, 2023-2030 ($)
Figure 40: Venezuela GDP – Composition of 2023, By Sector of Origin
Figure 41: Venezuela Export and Import Value & Volume, 2023-2030 ($)
Figure 42: Argentina Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 43: Argentina GDP and Population, 2023-2030 ($)
Figure 44: Argentina GDP – Composition of 2023, By Sector of Origin
Figure 45: Argentina Export and Import Value & Volume, 2023-2030 ($)
Figure 46: Ecuador Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 47: Ecuador GDP and Population, 2023-2030 ($)
Figure 48: Ecuador GDP – Composition of 2023, By Sector of Origin
Figure 49: Ecuador Export and Import Value & Volume, 2023-2030 ($)
Figure 50: Peru Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 51: Peru GDP and Population, 2023-2030 ($)
Figure 52: Peru GDP – Composition of 2023, By Sector of Origin
Figure 53: Peru Export and Import Value & Volume, 2023-2030 ($)
Figure 54: Colombia Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 55: Colombia GDP and Population, 2023-2030 ($)
Figure 56: Colombia GDP – Composition of 2023, By Sector of Origin
Figure 57: Colombia Export and Import Value & Volume, 2023-2030 ($)
Figure 58: Costa Rica Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 59: Costa Rica GDP and Population, 2023-2030 ($)
Figure 60: Costa Rica GDP – Composition of 2023, By Sector of Origin
Figure 61: Costa Rica Export and Import Value & Volume, 2023-2030 ($)
Figure 62: Europe Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 63: U.K Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 64: U.K GDP and Population, 2023-2030 ($)
Figure 65: U.K GDP – Composition of 2023, By Sector of Origin
Figure 66: U.K Export and Import Value & Volume, 2023-2030 ($)
Figure 67: Germany Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 68: Germany GDP and Population, 2023-2030 ($)
Figure 69: Germany GDP – Composition of 2023, By Sector of Origin
Figure 70: Germany Export and Import Value & Volume, 2023-2030 ($)
Figure 71: Italy Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 72: Italy GDP and Population, 2023-2030 ($)
Figure 73: Italy GDP – Composition of 2023, By Sector of Origin
Figure 74: Italy Export and Import Value & Volume, 2023-2030 ($)
Figure 75: France Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 76: France GDP and Population, 2023-2030 ($)
Figure 77: France GDP – Composition of 2023, By Sector of Origin
Figure 78: France Export and Import Value & Volume, 2023-2030 ($)
Figure 79: Netherlands Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 80: Netherlands GDP and Population, 2023-2030 ($)
Figure 81: Netherlands GDP – Composition of 2023, By Sector of Origin
Figure 82: Netherlands Export and Import Value & Volume, 2023-2030 ($)
Figure 83: Belgium Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 84: Belgium GDP and Population, 2023-2030 ($)
Figure 85: Belgium GDP – Composition of 2023, By Sector of Origin
Figure 86: Belgium Export and Import Value & Volume, 2023-2030 ($)
Figure 87: Spain Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 88: Spain GDP and Population, 2023-2030 ($)
Figure 89: Spain GDP – Composition of 2023, By Sector of Origin
Figure 90: Spain Export and Import Value & Volume, 2023-2030 ($)
Figure 91: Denmark Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 92: Denmark GDP and Population, 2023-2030 ($)
Figure 93: Denmark GDP – Composition of 2023, By Sector of Origin
Figure 94: Denmark Export and Import Value & Volume, 2023-2030 ($)
Figure 95: APAC Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 96: China Vascular Access Devices Market Value & Volume, 2023-2030
Figure 97: China GDP and Population, 2023-2030 ($)
Figure 98: China GDP – Composition of 2023, By Sector of Origin
Figure 99: China Export and Import Value & Volume, 2023-2030 ($) Vascular Access Devices Market China Export and Import Value & Volume, 2023-2030 ($)
Figure 100: Australia Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 101: Australia GDP and Population, 2023-2030 ($)
Figure 102: Australia GDP – Composition of 2023, By Sector of Origin
Figure 103: Australia Export and Import Value & Volume, 2023-2030 ($)
Figure 104: South Korea Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 105: South Korea GDP and Population, 2023-2030 ($)
Figure 106: South Korea GDP – Composition of 2023, By Sector of Origin
Figure 107: South Korea Export and Import Value & Volume, 2023-2030 ($)
Figure 108: India Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 109: India GDP and Population, 2023-2030 ($)
Figure 110: India GDP – Composition of 2023, By Sector of Origin
Figure 111: India Export and Import Value & Volume, 2023-2030 ($)
Figure 112: Taiwan Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 113: Taiwan GDP and Population, 2023-2030 ($)
Figure 114: Taiwan GDP – Composition of 2023, By Sector of Origin
Figure 115: Taiwan Export and Import Value & Volume, 2023-2030 ($)
Figure 116: Malaysia Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 117: Malaysia GDP and Population, 2023-2030 ($)
Figure 118: Malaysia GDP – Composition of 2023, By Sector of Origin
Figure 119: Malaysia Export and Import Value & Volume, 2023-2030 ($)
Figure 120: Hong Kong Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 121: Hong Kong GDP and Population, 2023-2030 ($)
Figure 122: Hong Kong GDP – Composition of 2023, By Sector of Origin
Figure 123: Hong Kong Export and Import Value & Volume, 2023-2030 ($)
Figure 124: Middle East & Africa Vascular Access Devices Market Middle East & Africa 3D Printing Market Value & Volume, 2023-2030 ($)
Figure 125: Russia Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 126: Russia GDP and Population, 2023-2030 ($)
Figure 127: Russia GDP – Composition of 2023, By Sector of Origin
Figure 128: Russia Export and Import Value & Volume, 2023-2030 ($)
Figure 129: Israel Vascular Access Devices Market Value & Volume, 2023-2030 ($)
Figure 130: Israel GDP and Population, 2023-2030 ($)
Figure 131: Israel GDP – Composition of 2023, By Sector of Origin
Figure 132: Israel Export and Import Value & Volume, 2023-2030 ($)
Figure 133: Entropy Share, By Strategies, 2023-2030* (%) Vascular Access Devices Market
Figure 134: Developments, 2023-2030* Vascular Access Devices Market
Figure 135: Company 1 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 136: Company 1 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 137: Company 1 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 138: Company 2 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 139: Company 2 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 140: Company 2 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 141: Company 3 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 142: Company 3 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 143: Company 3 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 144: Company 4 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 145: Company 4 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 146: Company 4 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 147: Company 5 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 148: Company 5 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 149: Company 5 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 150: Company 6 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 151: Company 6 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 152: Company 6 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 153: Company 7 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 154: Company 7 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 155: Company 7 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 156: Company 8 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 157: Company 8 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 158: Company 8 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 159: Company 9 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 160: Company 9 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 161: Company 9 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 162: Company 10 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 163: Company 10 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 164: Company 10 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 165: Company 11 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 166: Company 11 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 167: Company 11 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 168: Company 12 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 169: Company 12 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 170: Company 12 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 171: Company 13 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 172: Company 13 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 173: Company 13 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 174: Company 14 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 175: Company 14 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 176: Company 14 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
Figure 177: Company 15 Vascular Access Devices Market Net Revenue, By Years, 2023-2030* ($)
Figure 178: Company 15 Vascular Access Devices Market Net Revenue Share, By Business segments, 2023 (%)
Figure 179: Company 15 Vascular Access Devices Market Net Sales Share, By Geography, 2023 (%)
 Email
Email Print
Print

